
L Wolterbeek Muller and F J De Heer Physica 48 345 (1970)Į Horsdal-Pedersen, C L Cocke and M Stockli Phys. N V de Castro Faria, F L Freire and A G de Pinho Phys. Data Nucl Data Tables 14 177 (1974)į W Byron and C J Joachain Phys. The need and importance for accurate atomic and molecular.

53 245202 (2020)Į Clementi and C Roetti At. Keywords: charge transfer, electron capture, cross sectionscaling formula, heavy ion, tungsten. S Samaddar, D Jana, K Purkait and M Purkait J. Write down the - decay equation and determine the maximum kinetic energy of the electrons emitted. Electron capture has the same effect on the nucleus as does positron emission: The atomic number is decreased by one and the mass number does not change. It is estimated that this probability is. R Okasaka, K Kawabe, S Kawamoto, M Tani, H Kuma, T Iwai, K Mita and A I Wamae Phys. These eigenfunctions satisfy the appropriate equation for all internuclear separations if the velocity of relative motion is zero but they do not as they. Like positron emission, electron capture occurs for proton-rich nuclei that lie below the band of stability. The method is applied to developing a formula for the relative probability for capture into an excited state. J Gao, Z Hu, Y Wu, J Wang, N Sisourat and A Dubois Matter Radiat. Writing Positron Decay and Electron Capture Equations 1) The nuclide that decays is the one on the left-hand side of the equation. J W Gao, Y Wu, J G Wang, N Sisourat and A Dubois Phys. Theoretical investigations on state-selective total and angular differential cross sections for single-electron capture in collision of \(He^\)ffler et al Phys. Obviously, for any of the modes to occur the corresponding Q-value has to be larger than zero. For smaller Q-values only electron capture will occur. If Q is larger than, both electron capture and β+-decay are competitive processes, because they lead to the same daughter nucleus. The Q-values of the last two reactions are related by Therefore, the mass difference between both has to be larger than for β+ -decay to occur.

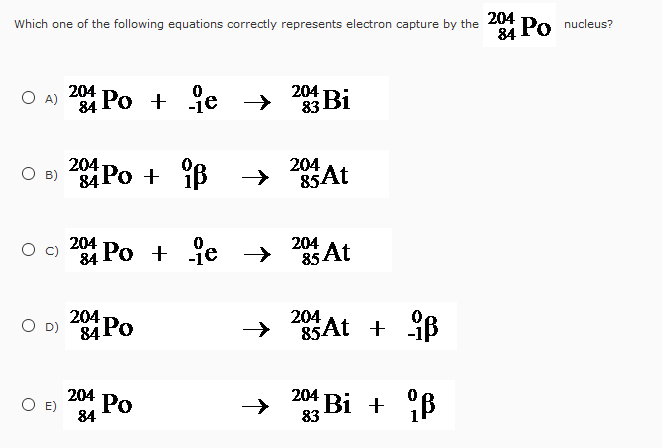
It represents the available energy in a nuclear transition.īecause all masses are given for atoms, this decay requires the rest mass of two electrons. The Q-value corresponds exactly to the mass difference between the mother and the daughter atom. The corresponding decay energies are given by the following relations, where denotes the mass of the neutral atom (not the nucleus) : 11 Altmetric Metrics Abstract The 229 Th nucleus has an isomeric state at an energy of about 8 eV above the ground state, several orders of magnitude lower than typical nuclear excitation energies. This process will reduce the atomic number by one and not changed the atom's mass. The result is that a proton will combine with this electron and a neutron is formed. Covered is a quick definition, and a radioactive decay formula. Electron capture occurs when an inner-orbital electron (negatively charged) is captured by the nucleus (positively charged). The other decay modes are understood in an analogous way. In this chemistry lesson, review electron capture, which is a form of radioactive decay.
On the quark level respectively, see Figure 1. A fully consistent calculation of muon capture and beta decay rates is. The basic underlying mechanism for (1) is given by Atomic and nuclear parameters of single electron capture decaying nuclides.

This results in three possible decay modes: Beta decay is a nuclear transition, where the atomic number Z of the nucleus changes by one unit, while atomic mass A remains the same.


 0 kommentar(er)
0 kommentar(er)
